As reviewed elsewhere, peripheral-nerve rerouting
has considerable potential to restore some function after SCI. In the
previously discussed procedures, peripheral nerves emanating from the
cord above the injury site are surgically rerouted and connected to
those below the injury site. This reestablishes a functional neuronal
connection from the brain to a paralysis-affected muscle. For example, a
still functional nerve to the rib cage can be rerouted and connected to
a paralysis-affected nerve that controls urination or, alternatively, a
leg muscle. Many rerouting permutations exist, which have restored some
function at most levels of injury.
However, the procedures described here are
fundamentally different in that the rewired nerves are both below
the injury site. Nevertheless, through skin stimulation, this
below-the-injury rewiring can trigger voluntary bladder and bowel
function.
The driving force behind the development of these
function-restoring procedures has been Dr. Chuan-Guo Xiao.
 Although
originally from and currently working in China, he spent many years in
the US fine-tuning his methodology at the New York University School of
Medicine. In fact, his pioneering work was first funded by PVA.
According to Xiao, “Without the first two grants from PVA, I don't think
I could have gotten the two big grants from the National Institutes of
Health, which allowed me to transfer the idea from laboratory bench to
bedside.” He adds “I have been really grateful to PVA for funding an
idea from a Chinese urology fellow with even worse English than now.”
Although
originally from and currently working in China, he spent many years in
the US fine-tuning his methodology at the New York University School of
Medicine. In fact, his pioneering work was first funded by PVA.
According to Xiao, “Without the first two grants from PVA, I don't think
I could have gotten the two big grants from the National Institutes of
Health, which allowed me to transfer the idea from laboratory bench to
bedside.” He adds “I have been really grateful to PVA for funding an
idea from a Chinese urology fellow with even worse English than now.”
Description of Procedures
Although there are a number of nerve-reconnection
possibilities, Xiao frequently cuts the lumbar-level L5 ventral nerve
root and connects it end-to-end to a cut sacral-level S3 (or S2) ventral
nerve root, which innervates the bladder. (The ventral and dorsal roots
contain nerves that leave and enter the spinal cord, respectively).
After the axons within this surgically connected nerve are given the
time to regenerate to the target site, the patient can initiated voiding
by scratching or gently squeezing for about 10 seconds their legs or
buttocks, i.e., the skin associated with the L5 dermatome.
Basically, these actions trigger a sensory signal
that enters the cord via the L5-dorsal root, in turn, stimulating nerves
that leave the cord through the L5-ventral roots now connected to the
bladder-controlling S3-ventral nerve root. Provided this area of
rerouting is undamaged, the procedure is suitable for most injury
levels. Because the procedure does not restore bladder sensation,
individuals must consciously initiate the triggering process to urinate.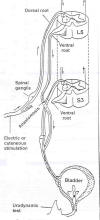
In 2003 and 2006 articles, Xiao reported the
results of treating 15 patients with ASIA-A-complete injuries with the
procedure [ASIA-impairment scale classifies injuries from grade A
(complete) to grade E (recovery)]. Injuries ranged from cervical C4 to
thoracic T12; in other words, all were above the nerve-rerouting area.
Age ranged from 25 to 55 (mean 39) years, and the time between injury
and surgery averaged 6.8 years. Patients were monitored for three years.
Of the 15 patients studied, 10 recovered
bladder-storage and -emptying function starting about a year after
surgery, the time it takes for neuronal axons to grow the 150
millimeters (~ 6 inches) to their target site. Residual urine decreased
from 332 to 31 milliliters, and urinary-tract infections became
negligible. In addition to these 10, two other patients recovered
partial function. These two required electrical stimulation of the skin
to initiate voiding, and, although residual urine volume was less, they
still retained over 100 milliliters. Of the three remaining patients,
one was lost to follow-up, and two did not accrue benefits, apparently
due to poor rerouting connections. Overall, there were no significant
short- or long-term complications.
Before surgery, six of the 12 patients who
eventually recovered some bladder control had elevated serum creatine
levels, an indicator of kidney problems. A year and half after the
procedure, their creatine levels returned to normal. In addition,
patients who regained bladder control also regained bowel control.
In a 2010 update posted on the SCI CareCure
discussion forum, Xiao indicated that since 2000 he and his colleagues
have cumulatively treated 350+ patients with SCI and 1,500+ patients
with spina bifida – a birth defect which results in an incompletely
developed spinal cord. Overall success rate exceeded 80%. In addition
to restoration of bladder and bowel function, he noted that 20-25% of
the patients regained some sexual functioning. He believes this sexual
improvement is due to the overall enhancement of the patients’ physical
condition after bladder and bowel function have been normalized.
To further disseminate his function-restoring
procedures, Xiao has trained numerous neurosurgeons in North America and
Europe, including the following:
Michigan: As reported on the National
Institutes of Health’s clinical trial registry and elsewhere, Drs.
Kenneth Peters and colleagues initiated a study evaluating Xiao’s
procedure in 12 subjects with either SCI (3) or spina bifida (9).
Preliminary results indicate that bladder and bowel function was
improved in many of the subjects. Peters emphasized, however, that
careful follow-up will be needed to understand the rerouting procedure’s
ultimate impact.
Louisiana: Drs. John Mata and Ravish
Patwardhan used the procedures to restore bladder function in a
seven-year-old girl who had been shot five years earlier.
Florida: Dr. Yves Homsy and
colleagues (Florida) have initiated a three-year study of the procedures
in children with spina bifida and SCI.
It should be noted that outside of China, the
availability of the procedure is primarily limited to a research
protocol.
Conclusion
As someone who has been involved in disability
research for many years, I’m amazed by how many promising,
function-restoring therapies are emerging throughout the world for SCI,
a disorder once considered so hopeless its cure was called the “Holy
Grail” of neurological research. Although none are an all-encompassing
“cure,” the dam is slowly, but inevitably, crumbling. It may be only a
small therapeutic trickle now, but it will become a flood. What is
especially promising in this case is the international collaboration
involved in developing and disseminating the procedures. Because SCI
bows to no flag, we need more of such collaboration.