Elsewhere, we have discussed the neuroprotective
potential of estrogen, a female-associated
hormone also produced by men at lower levels. Estrogen inhibits a
variety of neuron-damaging physiological processes that occur after
spinal cord injury (SCI) and may promote functional recovery.
This update will specifically review
progesterone-related neuroprotection. Although called the “pregnancy
hormone,” it is also synthesized to a lesser degree by men. Because
women possess higher levels of these hormones, when it comes to
neurological trauma, they are probably the stronger sex.
Progesterone
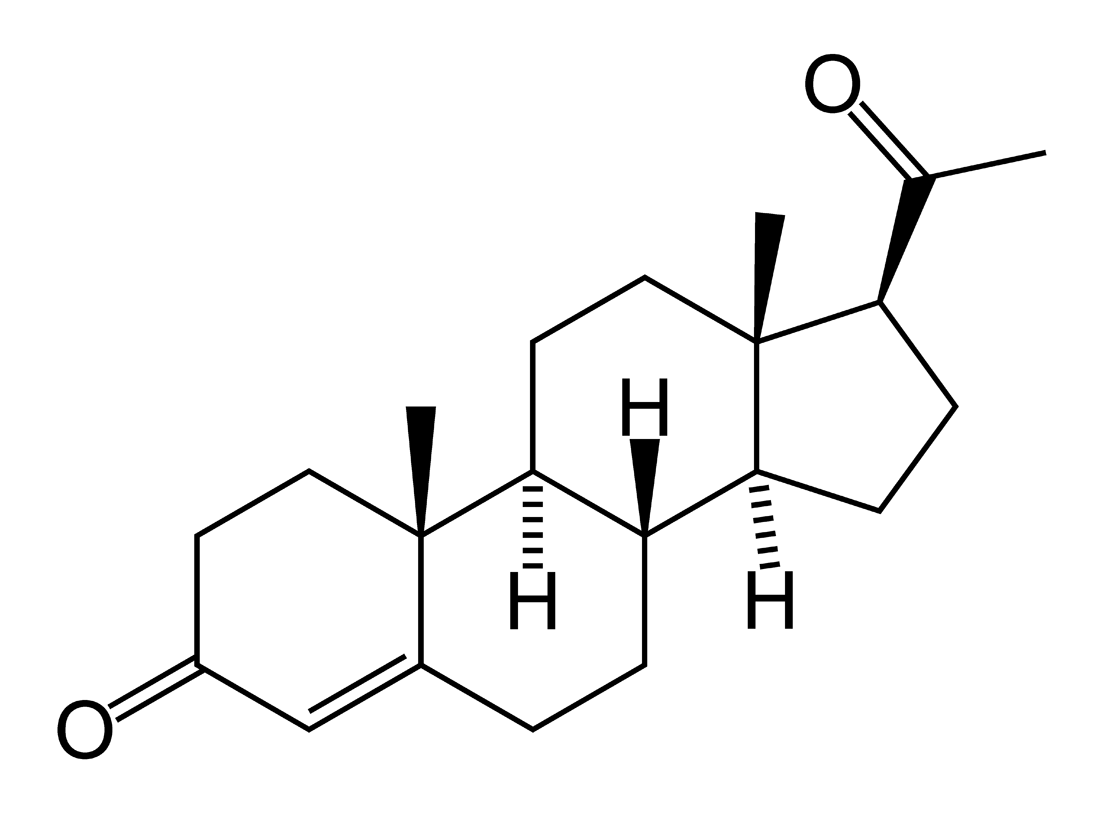
Progesterone is synthesized from cholesterol by the
ovaries, adrenal glands, and placenta. In the menstrual cycle,
progesterone levels are relatively low before ovulation and elevated
afterwards. Hormone levels are much higher throughout pregnancy, drop to
low levels after birth and during lactation, and recede after menopause.
In men, progesterone is produced by the testes and, paradoxically, is
the biochemical precursor to the defining male hormone testosterone.
In part by affecting the expression of other
body-regulating hormones, progesterone exerts many biological influences
throughout the body above and beyond its more well-known effects in
reproduction. Overall, our optimal functioning is dependent upon a
complex, interacting hormonal milieu, whose composition is dependent on
many factors, including gender, age, diet, life style, and overall
health.
Neurosteroid
With paradigm-expanding implications, progesterone
is also produced by and influences the nervous system and, as such, has
been termed a “neurosteroid.” Due to this localized synthesis,
nervous-tissue progesterone levels are not necessarily a function of
plasma levels of the hormone produced by more traditional sources.
Neurons and neuronal support cells (called glia) actually have unique
progesterone receptors on their outer membrane surface. Like a key
fitting in a lock, progesterone’s interactions with these receptors can
initiate complex, nervous-system-unique biological responses. Although
these responses are only beginning to be understood, they seem to
enhance neuronal health and viability.
Many studies suggest that progesterone treatment is
neuroprotective after trauma by limiting the loss of neuronal tissue
and, as a consequence, preserving function. Because membrane-soluble
progesterone can readily diffuse cross the blood-brain-barrier,
externally administered progesterone has the ability to reach the
nervous system. As a result, it has the opportunity to interact with the
various progesterone receptors on neuronal cells, shifting the
nervous-system environment into a more neuroprotective mode.
Although we must be careful in extrapolating
results to humans, progesterone neuroprotection has been documented in
numerous animal models of neurological disorders, including traumatic
brain injury (TBI) and spinal cord dysfunction, such as SCI, multiple
sclerosis (MS), and amyotrophic lateral sclerosis (ALS). For example, in
animal models of MS, progesterone treatment lessens disease severity,
reduces inflammation, and restores the conduction-promoting, insulating
myelin sheath surrounding neurons (see below). In ALS animal models, the
hormone inhibits the degeneration of motor neurons
TBI
Probably the most research has been directed to TBI,
partly because early studies suggested gender differences in recovery
after injury. For example, reproductive-cycling female rats with high
progesterone levels have less post-injury cerebral edema (swelling) than
male rats with inherently low progesterone. Pseudopregnant rats (a
pregnancy-like condition), whose progesterone levels are especially
high, had little post-injury edema.
Several clinical trials have examined
progesterone’s neuroprotective potential in humans. In the first, Emory
University investigators (Atlanta, GA) evaluated outcomes in a 100
acutely injured subjects treated with either intravenous progesterone or
placebo for three days. Thirty-days post injury, progesterone treatment
when compared to controls 1) reduced mortality in the severely injured,
and 2) and improved functional outcomes in the moderately injured.
In a second trial, Chinese researchers randomized
159 patients with severe TBI to receive five days of either progesterone
or placebo injections intramuscularly. Six months later,
progesterone-treated patients showed greater functional improvement and
lower mortality.
SCI
TBI research findings often, but not always, have
relevance to SCI. Although human studies are lacking, extensive animal
research supports progesterone’s neuroprotective potential for SCI.
Given the complex physiological cascade that occurs after injury, there
are many interacting, biological processes that progesterone could
target. For example, studies suggest that post-injury progesterone
treatment:
Remyelination
In addition to the aforementioned, evidence
indicates that progesterone promotes the post-injury remyelination of
neuronal axons. Myelin is the fatty insulating material enveloping
axons, i.e., the fibers that conduct electrical impulses away from the
neuron’s cell body to other nerves or muscles. When axons are
demyelinated, channels between the inside and outside of the axon are
exposed, in turn, causing disruption in the ionic equilibrium needed for
neural transmission. Although most associated with MS, demyelination
frequent occurs after SCI. Intact neurons may still traverse the injury
site, but because they have lost their insulation, they no longer
conduct. In theory, therapies that help restore the myelin sheath should
re-establish some function-restoring conduction.
In the spinal cord, myelin is produced by
oligodendrocytes, a neuronal support cell which is formed through
the differentiation of oligodendrocyte precursor cells. Because
oligodendrocytes are extremely sensitive to injury, much-needed
remyelination capability is lost. Evidence indicates that progesterone
treatment enhances the proliferation of the normally quiescent precursor
cells into mature, myelin-producing oligodendrocytes, enhancing the
conduction of injury-waylaid neurons.
It is important to note when discussing potential
restorative treatments, only a relatively small percentage of intact,
functioning neurons are needed to regain significant function. In other
words, if progesterone-triggered remyelination can jump-start a few
neurons, significant function may accrue.
Hindlimb Functional Recovery
In spite of this promising research, animal studies
directed toward recovery of function after SCI are limited and ambiguous
in results. In one study, scientists at the Henry Ford Health Sciences
Center (Detroit, MI) evaluated the effect of progesterone treatment in
rats with SCI produced by contusion, the sort of injury common in
humans. After injury, rats received progesterone injected into the body
cavity periodically for five days. Compared to controls,
six weeks after injury, progesterone-treated
animals recovered more function and had greater tissue preservation at
the injury site.
However, research by University of
Kentucky investigators (Lexington, KY) could not replicate these
benefits. Specifically, after an experimental contusion injury, rats
were treated with progesterone with several dosing regimens for up to 14
days. Three weeks after injury, no significant improvement in hindlimb
function was observed between the progesterone-treated and control
animals.
Conclusion
Although the jury is still out on progesterone’s
true SCI neuroprotective potential, a growing foundation of evidence
suggests the topic warrants further investigation. Given the paucity of
real-world SCI therapies, the idea that a hormone, which has been shown
to be not only produced by the nervous system but to influence
nervous-system viability, may help repair a damaged spinal cord makes a
lot of sense.
References: An extensive list of references
is posted at
www.sci-therapies.info (Click on Bibliography in the Table of
Contents and scroll down to “Pharmaceutical Approaches for Acute SCI.”)
Adapted from article appearing in June 2011 Paraplegia News (For subscriptions,
call 602-224-0500) or go to
www.pn-magazine.com.
TOP