Part 1 introduced
the pineal gland from both a scientific and metaphysical viewpoint. We
will now discuss how both cervical spinal cord injury (SCI) and multiple
sclerosis (MS) are associated with pineal dysfunction and how the
gland’s key hormonal product melatonin may be neuroprotective after
injury.
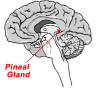
In brief review, the pineal gland is a small,
pea-shaped organ located in the middle of the head that secretes
melatonin. This hormone is released into the bloodstream and
cerebrospinal fluid where it is transported throughout the body.
Affecting many bodily functions, melatonin secretion is closely
correlated to our sleep-wake cycle and regulates sexual/reproductive
function.
Pineal functioning tends to diminish over time, and
melatonin-compromising calcification of the gland is not uncommon in
adults.
SCI
In spite of its mid-brain location, the pineal
gland is innervated by nerves that come out of the cervical spinal cord.
As demonstrated by several researchers, cervical, but not lower-level,
injuries compromise the pineal gland and its melatonin production:
For example, Dr. Y. Li and colleagues
(China) compared the daily diurnal rhythm of melatonin secretion in
individuals with chronic cervical injuries with subjects with lower
level injuries. In the cervical-injury group, melatonin levels were
low, and no diurnal rhythm was observed. In contrast, in subjects with
lower-level injuries, melatonin levels and cycles were maintained.
Dr. Jamie Zeitzer et al, Harvard University
and the Brockton/West Roxbury VA Medical Center (Massachusetts) compared
melatonin production in three subjects with chronic cervical injuries
(C6, C6/7, & C4 injuries) with two individuals with thoracic injuries.
The investigators concluded that neurologically complete cervical SCI
results in a total loss of pineal melatonin production.
Although sleep quality in subjects with thoracic
injuries was similar to able-bodied individuals, it was compromised in
subjects with cervical injuries who lacked nocturnal melatonin
production. For example, the onset of REM sleep averaged 220 minutes in
the subjects with cervical injuries compared to only 34 minutes for
those with thoracic injuries (REM or rapid-eye-movement sleep is
associated with dreaming). The investigators suggested that melatonin
supplementation might restore normal sleep in individuals with cervical
SCI
Neuroprotection
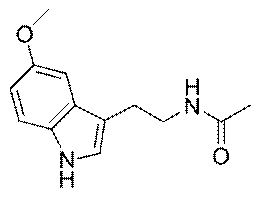
Melatonin is also a powerful antioxidant that
protects cells from damaging oxidation. Specifically, it is a highly
efficient scavenger of free radicals, molecules which seek out electrons
to achieve a more stable energetic state. Melatonin’s structural
affinity for fat or lipid allows it to diffuse through the lipophilic
cell membranes and scavenge free radicals within the cell.
Melatonin
Free radicals mediate damage after
acute SCI. Following the initial mechanical injury, a complicated
physiological chain reaction generates free-radicals, which, in turn,
steal electrons from lipids in nearby neuronal and axonal membranes.
Called lipid peroxidation, this process results in further cell
death.
Like the commonly
administered methylprednisolone, animal
studies indicate that melatonin inhibits lipid peroxidation and various
injury-aggravating inflammatory processes. Sample studies include:
Dr. Toru Fujimoto
and colleagues (Japan) examined melatonin’s neuroprotective effects in
rats with SCI produced by placing a weight on the exposed cord.
Melatonin was injected into the body cavity before and after injury.
Compared to controls, the melatonin-treated rats had less lipid
peroxidation, smaller injury-site cavities, and retained more hind-limb
function.
Dr. S. F. Erten
et al (Turkey) assessed neuroprotection in rabbits with
spinal-cord ischemia generated by clamping down on blood vessels serving
the area. Melatonin-treated rabbits had less lipid peroxidation.
Dr. Jin-bo Liu
and associates (China) examined melatonin protection in rats with
injuries created by dropping a weight on the exposed cord. The
investigators concluded that “melatonin can prevent oxidative damage,
reduce neurological deficit, and facilitate the recovery from” SCI.
Drs. Tiziana Genovese
et al (Italy) evaluated melatonin in rats with injury produced by
clipping the cord. The results indicated “that melatonin can exert
potent anti-inflammatory effects” and enhanced hind-limb functional
recovery.
Dr. Suleyman Cayli
et al (Turkey) compared the effectiveness of 1)
melatonin, 2) methylprednisolone, and 3) a combination of the two drugs.
Improvements were noted in all three treatment groups, including
enhanced neuronal conduction, recovery of motor function, decreased
lipid peroxidation, and improved injury-site structural integrity. The
combination treatment was best at inhibiting lipid peroxidation.
The aforementioned experiments
injected high levels of melatonin into the body. However, research by
Dr. O. Ates and colleagues (Turkey) suggest that even
physiological background levels may be important. Specifically, the
investigators assessed the effect of removing the rat’s pineal gland
and, hence, the melatonin source before injury. Because pinealectomy
increased post-injury lipid peroxidation, the investigators concluded
that the reduction in endogenous melatonin made rats more vulnerable to
trauma.
These findings have considerable
relevance to humans. They suggest that individuals with less
pineal function may have more neurological damage after injury.
Because pineal functioning and melatonin
production tends to diminish with age, the researchers concluded
“endogenous melatonin level may make the age of the patient an important
parameter for recovery” after SCI.
Because drinking-water fluoridation also impairs
pineal melatonin production, it is possible that our efforts to fight
cavities have resulted in more paralysis for individuals sustaining
injuries.
MS
Unlike cervical SCI which leads to pineal
dysfunction, the converse may be true for MS. Because pineal dysfunction
and, in turn, low melatonin secretion are correlated with MS symptoms,
pineal failure may predispose one to MS. For example, Dr. Reuven
Sandyk (New York) has stated “Dysfunction of the pineal gland can
explain a far broader range of biological phenomena associated with MS,
and therefore the pineal gland should be considered the pivotal mover of
the disease.” In his model, various environmental, hormonal, and genetic
factors lead to pineal dysfunction. In turn, the resulting low melatonin
levels promote MS-associated physiology, such as the disorder’s
characteristic neuronal demyelination. He believes therapeutically
focusing on demyelination distracts us from dealing with the disorder’s
more primary causes.
Sandyk suggests that MS severity may be related to
the degree of pineal failure.
In the case of relapsing-remitting MS, spontaneous
remission of MS symptoms may be mediated through the pineal gland’s
renewed melatonin production. However, in the case of chronic
progressive MS, more extensive pineal dysfunction and calcification
prevents the remission.
Clearly, MS is associated with pineal
calcification. For example, one study showed 100% of individuals with MS
who were consecutively admitted to a hospital had pineal calcification
compared to only 43% for similar-aged controls with other neurological
disorders. In addition, groups that have a low MS incidence (e.g.,
African Americans, Japanese) also have less pineal calcification.
Conclusion
Called the seat of the soul by French
philosopher René Descartes, the tiny pineal gland has more mythological
mystique than virtually any other body part. Although the mystique is a
matter of metaphysical speculation, the gland’s profound role in
influencing our physiology is scientifically documented. Unfortunately,
on top of paralysis, people with SCI and MS also may have to deal with
all the mind-body-spirit ramifications of a dysfunctional pineal gland.
Adapted from article appearing in October 2009 Paraplegia News (For subscriptions,
call 602-224-0500) or go to
www.pn-magazine.com.
TOP