In an earlier aromatherapy
article, I explained how nasal tissue captures odor molecules; this,
in turn, triggers signals to be sent to the brain that affect the entire
body. Due to the tissue’s unique characteristics, it possesses
extraordinary regenerative potential, which many scientists believe can
be exploited to restore function after spinal cord injury (SCI).
Building upon a foundation of animal
experimentation, scientists in Portugal, Australia, and China, have
begun to transplant olfactory tissue or cells into the injury site of
humans with chronic injuries. Part 1 of this two-part article will
review olfactory tissue’s unique properties, laying the foundation for
Part 2’s summary of Portugal’s Dr. Carlos Lima’s pioneering work
in humans.
Introduction:
Olfactory tissue covers about 2.5 centimeters (1
cm = .39 inches) of the upper 1-cm surface of each nasal cavity.
Integrated into this tissue are bipolar olfactory neurons, which,
starting at the tissue’s surfac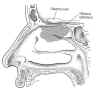 e,
are composed of 1) dendrites, hair-like projections that receive
informational molecules; 2) the olfactory knob from which the dendrites
are attached; 3) the cell body, containing the neuron’s nucleus and
metabolic center; and 4) the signal-conducting axon. Receptors on the
dendrite surface capture inhaled odor molecules, which, like a key
turning a lock, trigger nerve impulses to the brain through the axon.
e,
are composed of 1) dendrites, hair-like projections that receive
informational molecules; 2) the olfactory knob from which the dendrites
are attached; 3) the cell body, containing the neuron’s nucleus and
metabolic center; and 4) the signal-conducting axon. Receptors on the
dendrite surface capture inhaled odor molecules, which, like a key
turning a lock, trigger nerve impulses to the brain through the axon.
The axons come together to form bundles (fascicles)
that are enveloped by olfactory ensheathing cells (OEC’s), a
special type of glial or neuronal support cell that guides the axon and
supports its elongation. The bundles travel to the base of the tissue
and cross over to the cranial cavity through a perforated area of bone
named the cribrifor m
plate. They then enter the brain’s olfactory bulb, a relay station
where they make connections with second-order neurons that lead to other
brain areas via the olfactory tract.
m
plate. They then enter the brain’s olfactory bulb, a relay station
where they make connections with second-order neurons that lead to other
brain areas via the olfactory tract.
As a simple analogy, visualize an olfactory neuron
as a potbellied dachshund with a long tail sticking through a fence
hole. The dog’s side of the fence represents the nose’s olfactory
tissue, the tail side the brain’s olfactory bulb, and the fence the
cranial barrier. Except for the tail, the dog resides on the fence’s
olfactory-tissue side. The dog’s whiskers represent dendrites that are
attached to the dog’s head (i.e., olfactory knob), its potbelly
represents the nucleus-containing cell body, and its long tail
represents the axon.
When a small fly (i.e., the odor molecule)
stimulates the dog’s whiskers, his nose twitches, initiating a shake
(i.e., nerve impulse) that quickly descends down his body until his tail
wags on the other side of the fence. This wagging excites dogs that live
on the other side (i.e., second-order neurons), who, in turn, signal the
whole neighborhood (i.e., brain, then body).
Scientists are excited about olfactory tissue
because, unlike spinal cord tissue, it contains so many cells with
regenerative potential, including a source of renewable neurons,
progenitor stem cells, and remyelinating OEC’s.
Olfactory Neurons:
These are unique in many ways. For example, most
nerves are either a part of the central nervous system (CNS) - i.e.,
brain and spinal cord - or the peripheral nervous system (PNS), which
connects organs and extremities to the CNS. Each system’s cellular
environment is hostile to the other’s nerves. For example, injured
peripheral nerves will stop regrowing when they hit the spinal cord.
However, this classification is ambiguous for olfactory neurons, which
are comfortable in both the PNS and CNS.
In another example, olfactory neurons are the
body’s only surface neurons with direct access to the external
environment, i.e., the air we breathe.
Like all surface cells, they readily replicate and regenerate,
turning over every 60 days throughout life. In olfactory tissue, there
are always neurons in different stages of neurogenesis. As neurons
mature, they migrate from the base to the surface of the tissue and
replace mature neurons, which die through apoptosis, a form of
programmed cell death.
Olfactory Stem Cells:
The source of these new neurons is a pool of progenitor
stem cells that reside at the tissue’s base. Due to their
potential to differentiate into cells that can treat neurological
disorders, stems cells have been the focus of much research and also
controversy because they have often been isolated from fetal tissue, a
stigma olfactory-derived stem cells avoid.
Olfactory Ensheathing Cells:
Axonal regenerative potential is enhanced by
OEC’s, which 1) although they do not do so with olfactory neurons
themselves, produce insulating myelin sheaths around both growing and
damaged axons in the spinal cord, 2) secrete various growth-enhancing
neurotrophic agents, and 3) produce structural and matrix macromolecules
that lay the tracks for axonal elongation. Because of these features,
OEC’s promote axonal regrowth, including when implanted in areas that
normally do not readily regenerate, such as the spinal cord.
For example, OEC-remyelinated spinal cord axons have been shown
to penetrate the inhibitory glial scar at the injury site, and then to
migrate to their correct targets, restoring function.
For a severed axon attempting to grow through this
glial scar, it is the physiological equivalent of running the gauntlet,
in which the clubs preventing the axon’s passage are the glial
scar’s inhibitory molecules. Because of this gauntlet, the truncated
axon retreats into safety. So to speak, the implanted OEC’s provide an
insulating armor that enables the struggling axon to fend off the
inhibitory molecular clubs, pass through the gauntlet, and travel back
home in a more receptive environment.
In addition, although many structurally intact
neurons routinely circumvent the injury glial-scar, the majority of them
do not conduct because they have been demyelinated. By providing new,
conduction-restoring, myelin insulation, OEC’s once again come to the
rescue.
Because only a small amount of functional neurons
(10-15%) are needed to regain significant function, olfactory-tissue’s
regeneration-fostering properties cumulatively portend much promise for
SCI.
Animal Studies:
Many animal studies have documented olfactory
tissue’s potential to restore function after SCI.
Human Transplantation:
If the patient is the source of transplantation
material (i.e., called autologous grafting), immunosuppressive drugs
will not be needed to minimize tissue rejection. Patient tissue can be
obtained by a simple biopsy through the nostril, which will not affect
long-term olfactory capability. This procedure is clearly preferable to
penetrating the cranium to access the olfactory bulb, the OEC source in
much animal research.
Scientists have transplanted both OEC’s and
olfactory tissue into patients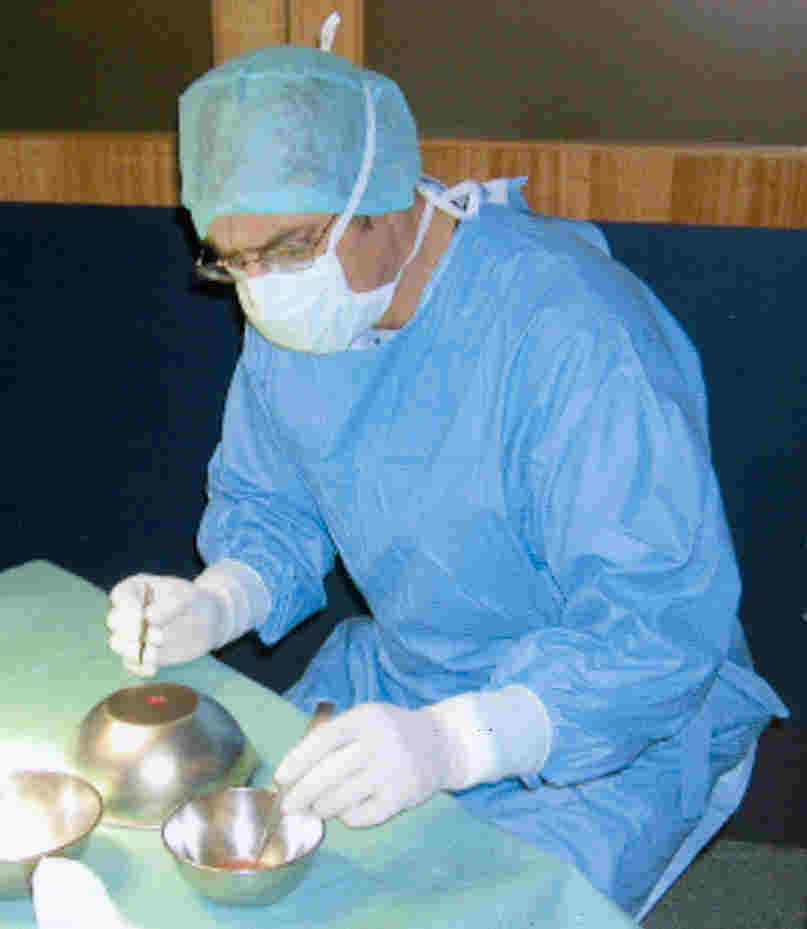 with SCI. For example, Portugal’s Dr. Lima implanted autologous
olfactory tissue back into the spinal cords of seven patients. Lima
believes that more than one cell type is needed to maximize regeneration
in the injured cord, including, in addition to OEC’s, neurons in
different developmental stages, and precursor stem cells. (photo: Lima
removes about 25% of the patient's olfactory tissue, which is then
minced and implanted into the spinal cord)
with SCI. For example, Portugal’s Dr. Lima implanted autologous
olfactory tissue back into the spinal cords of seven patients. Lima
believes that more than one cell type is needed to maximize regeneration
in the injured cord, including, in addition to OEC’s, neurons in
different developmental stages, and precursor stem cells. (photo: Lima
removes about 25% of the patient's olfactory tissue, which is then
minced and implanted into the spinal cord)
In contrast, in Australia Dr. Alan MacKay-Sim’s
team has implanted OEC’s previously isolated and cultured from the
patient back into the cord. Scientists, who by nature are concerned
about cause-and-effect mechanisms, like such an approach because it
reduces the number of confounding factors that could exert an effect.
China’s Dr. Hongyuan Huang offers a third
approach. He transplanted OEC’s isolated from fetal tissue into more
than 150 patients. Because of fetal tissue’s undifferentiated nature,
immunosuppression drugs have not been required so far.
Although these preliminary efforts are promising,
much still needs to be learned before a definitive judgment can be made
on the therapy’s true potential. For example, in some cases, the
surgery may result in the decompression of the spinal cord, which by
itself could result in functional recovery.
Conclusion:
Growing evidence indicates that olfactory tissue
and ensheathing cells have considerable potential to repair the
traumatically injured spinal cord. Based on this potential, scientists
have begun to treat humans with chronic injuries, including Portugal’s
Dr. Carlos Lima, whose work will be summarized in Part 2.
Adapted from article appearing in Paraplegia News, March, 2003 (For subscriptions, contact www.pn-magazine.com).