This
update specifically summarizes the potential of oscillating field
stimulation (OFS) to minimize neurological damage after acute SCI. OFS
was the focus of an article published in the Journal of Neurosurgery
Spine (January 2005) by Dr. Scott Shapiro and colleagues at Purdue
University (West Lafayette, IN).
OFS
therapy is based upon observations that appropriate electrical cues guide
and promote neuronal growth. For example, studies suggest that in early
development, naturally occurring voltage gradients channel nascent neurons
down the neural tube, the spinal cordís anatomical precursor. In addition,
studies indicate that 1) regenerating axons are attracted to an electric
field cathode (i.e., negative pole of applied field) and 2) such a field
may alter neuron-supporting glial cell density and organization within the
injury scar in a fashion that is less inhibitory to regeneration.
Because
implanting the cathode above or below the injury site will promote
neuronal growth only in one direction, the OFS device alternates polarity
every 15 minutes. With such a device, regeneration in both ascending and
descending neurons is stimulated.
OFS
therapy is only beneficial for acute injury. If device implantation is
delayed several months, regenerative benefits will not accrue.
Animal
Research
OFS
therapy is based on extensive research by Shapiroís colleague, Dr. Richard
Borgens, using dogs with naturally occurring SCI, often due to explosive
disk herniation that rapidly progresses into complete SCI. Borgenís
research includes two randomized, controlled trials in dogs (Borgens et
al. J Restorative Neurol Neurosci 5, 1993 and Borgens et al. J
Neurotrauma 16, 1999), which laid the foundation for the recent human
trial discussed below.
In Dr.
Borgenís 1999 dog study, OSF devices were implanted in 20 paraplegic dogs
and compared to 14 dogs with an implanted sham device. After six months,
overall improvement, measured by a variety of neurological assessments,
was greater in OFS-treated dogs.
Human
Study
Given
these results, the Food and Drug Administration (FDA) approved a Phase-1
clinical trial to assess safety in ten patients with complete injuries
ranging from the C5 to T10. The patientsí age ranged from 18 to 43 (median
age 23) and all but one were males. Six injuries were due to motor vehicle
accidents, two from falls, one from diving, and one from violence.
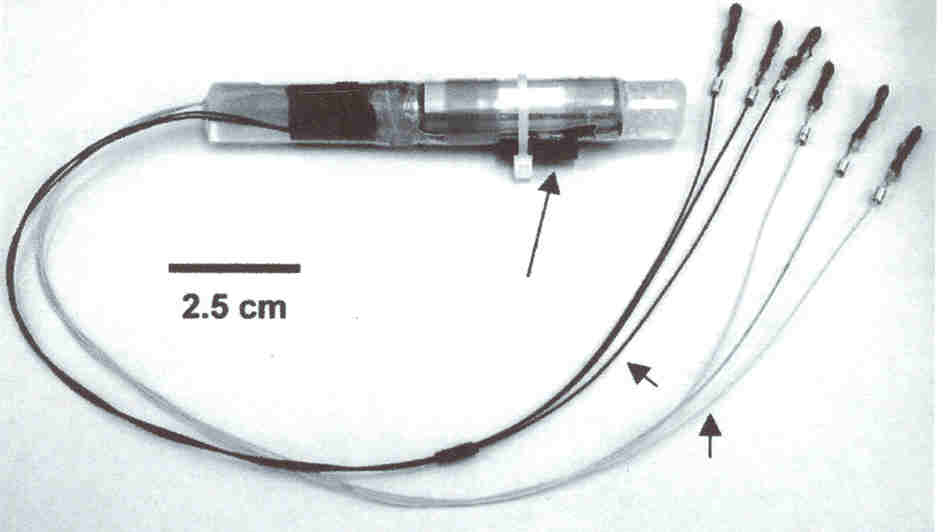
Within 18
days of injury, the cylindrical OFS device (11-centimeter long; 1-cm
diameter) was implanted in the patientsí paraspinous musculature below the
injury site to minimize pain or discomfort. Emanating from the device are
two sets of three electrodes. The electrodes in one set are sutured to the
spineís spinous process and right and left facet joints one segment above
the injury site, and the other leads are connected to the same points a
segment below the injury site. Simply stated, for a C6 injury, the
electrodes would be connected to the spine at the C5 and C7 level. The OFS
device was removed at 15 weeks.
Results
indicated that the procedure was safe, the goal of this Phase-1 trial.
A variety
of functional assessments was carried out at six months and one year after
implantation. All subjects demonstrated improved sensation, and some
regained significant motor or sexual function. However, because no
controls were included in this study designed to assess safety, overall
efficacy could not be directly evaluated as in the case of the
aforementioned dog studies. Although improvements were observed for most
subjects a year after implantation, some recovery is routinely noted
during this post-injury period. As such, because the study had no built-in
reference point, results were compared to patient improvements documented
in the third NASCIS (National Acute Spinal Cord Injury Study) trial. This
comparison strongly suggests that OFS therapy exerts beneficial effects
after acute injury in humans.
FDA has
improved additional testing in patients with acute SCI, and Purdue
University has licensed the technology to Andara Life Sciences, Inc.
Adapted from article appearing in October 2005 Paraplegia News (For subscriptions,
call 602-224-0500) or go to www.pn-magazine.com).
TOP