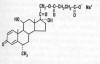
Methylprednisolone (MP) is a glucocorticoid steroid
(illustration) administered soon after spinal cord injury (SCI) in an
effort to minimize neurological damage. If you have sustained a SCI over
the past dozen years or so, you were probably treated with this drug.
Although it has become a de facto post-injury standard of care in
the U.S., many scientists are challenging MP’s effectiveness and
underlying scientific foundation.
MP Studies
Extensive animal research suggests MP reduces
post-injury neurological damage, in part, by inhibiting lipid
peroxidation, a biochemical process that mediates secondary damage to
the injured cord. A number of large MP-focused clinical trials have been
conducted, including the National Acute SCI Study (NASCIS) 1, 2, and 3.
In the NASCIS-2 study (NASCIS 1 generated no
significant results), 162 acutely injured patients received a high MP
dose consisting of an initial bolus of 30 milligrams (mg) per kilogram
(1 kg) of body weight. This was followed by infusion of 5.4 mg/kg/hour
for 23 hours. The patients were compared to 171 subjects given an
inactive placebo. Motor and sensory function was assessed at admission,
after six weeks, and after six months. Investigators concluded that
patients treated with MP within eight hours of injury had improved
neurological recovery.
Before the results were published, the National
Institutes of Health (NIH) disseminated them through announcements and
faxes to emergency-room physicians and the news media. Though this was
done to help newly injured patients as soon as possible, it essentially
created a standard of care before other experts could critically
evaluate the results.
In NASCIS 3, after receiving the same initial MP
dose within eight hours of injury, 499 acutely injured subjects were
randomized to receive the same rate of MP infusion as described above
for 24 or 48 hours (a third of patients received another drug).
Follow-up assessments were again carried out at six weeks and six
months. The investigators concluded that if MP is initially
administered within three hours of injury, the regimen should be
continued for 24 hours; if initiated three to eight hours after injury,
the regimen should be continued for 48 hours.
MP’s side effects included gastrointestinal
bleeding, wound infections, and delayed healing. The
NASCIS studies and other smaller studies suggest an increased incidence
of sepsis (blood-stream infection) and pneumonia in the MP-treatment
groups.
Critics
Reflecting Mark Twain’s statement “There are three
kinds of lies: lies, damned lies, and statistics,” many critics believe
that MP has been promoted as a standard of care for acute SCI based on
results generated through the use of questionable statistical
procedures. These doubters claim that NASCIS showed little if any
statistically significant benefits from high-dose MP, and modest
benefits were only demonstrated in a patient subgroup when data was
later micro-analyzed.
This controversy is not insignificant. For example,
a survey of participants at a 2001 Annual Canadian Spine Society meeting
indicated “75% of respondents were using MP either because everyone else
does or out of fear for failing do so.”
This society and the Canadian Neurosurgical Society
commissioned an expert review of the available MP data, which concluded
that there was insufficient evidence to support the use of MP as a
treatment standard or guideline, although weak clinical evidence
supports its use as a treatment option.
Other articles challenging MP’s use include:
1) Dr. Shanker Nesathurai (Boston) said that
neither NASCIS 2 nor 3 convincingly demonstrated MP’s benefits (J
Trauma 1998; 45[6]). “There are concerns about the statistical
analysis, randomization, and clinical benefits… Furthermore, the
benefits of this intervention may not warrant the possible risks.”
2) Dr. Deborah Short and colleagues (U.K.)
concluded: “The evidence produced...does not support the use of
high-dose methylpredinisolone in acute spinal-cord injury to improve
neurological recovery. A deleterious effect on early mortality and
morbidity cannot be excluded by this evidence.” (Spinal Cord,
2000; 38[5])
3) Dr. W.P. Coleman et al (Annapolis Md) strongly
criticized both NASCIS 2 and 3 for methodological weaknesses and the
lack of data that could be critically reviewed by others (J Spinal
Disord 2000; 13[3]). For example, they stated: “The
numbers, tables, and figures in the published reports are scant and are
inconsistently defined, making it impossible even for professional
statisticians to duplicate the analyses, to guess the effect of changes
in assumptions, or to supply the missing parts of the picture.
Nonetheless, even 9 years after NASCIS II, the primary data have not
been made public…These shortcomings have denied physicians the chance to
use confidently a drug that many were enthusiastic about and has left
them in an intolerably ambiguous position in their therapeutic choices,
in their legal exposure, and in their ability to perform further
research to help their patients.”
4) Dr. R.J. Hurlbert (Alberta, Canada) concluded:
“The use of methylprednisolone administration
in the treatment of acute SCI is not proven as a standard of care, nor
can it be considered a recommended treatment. Evidence of the drug's
efficacy and impact is weak and may only represent random events. In the
strictest sense, 24-hour administration of methylprednisolone must still
be considered experimental for use in clinical SCI. Forty-eight-hour
therapy is not recommended.” (J Neurosurg 2000; 93[1 suppl])
5) Dr. Tie Qian and colleagues (Newark, NJ)
suggested that high-dose MP may damage muscles through acute
corticosteroid myopathy (ACM) and that functional improvement attributed
to MP may merely be due to the recovery of muscle damage caused by this
extremely high MP dose (Med Hypothesis 2000; 55[5]). The
investigators noted that under the NASCIS 3 clinical protocol, a 75-kg
(165 pounds) acutely injured individual could receive nearly 22 grams of
MP, which is the “highest dose of steroids during a 2-day period for any
clinical condition.”
This is, indeed, a strong indictment. As a crude
analogy, consider this: I punch you forcefully in the arm and create a
bad bruise. I then claimed that the healing of the bruise was due to -
and not caused by - the punch. In this analogy, MP is the punch.
6) Further investigating this issue, Qian and
colleagues (Miami, Fla) compared five acutely injured patients who
received high-dose MP regimen with three control patients, who did not
meet the requirements for MP treatment (specifically, two gunshot
injuries and one who arrived at hospital too late) (Spinal Cord
2005; 43[4]). Muscle damage was assessed by biopsy and electromyography
(EMG - measures the amount of conduction signal reaching the muscle).
The biopsies and EMG data indicated that four of the five MP-treated,
but no control, patients had muscle damage consistent with ACM. The
investigators concluded that “the improvement of neurological recovery
showed in NASCIS may be only a recording of the natural recovery of ACM,
instead of any protection that MP offers to the injured spinal cord.”
Conclusion
Because I was a NIH division director when the
agency promulgated its MP-treatment policy, I know that it was a
conscientious decision in an effort to help those with acute injuries.
Unfortunately, we may have to revisit the policy, which could be
difficult given that changing the direction of entrenched public-health
policies is like an aircraft carrier making a U-turn. In other words; it
takes a long time.
Some of our most-distinguished NIH scientific
advisors built their reputations on this potentially flawed policy and
may be reluctant to advise the agency to adjust course. Nevertheless, we
need more clarity concerning the true benefits of this drug routinely
used to treat acute SCI.
TOP