1) Introduction
2) Dr. Carlos Lima's Insights
3) Insights from
Animal Studies: Stem Cells vs. OECs
INTRODUCTION
Laurance Johnston, Ph.D.
Under the traditional Hippocratic Oath, physicians swore to Asklepios,
the Greek god of medicine who healed people and made them immortal.
Asklepios’ healing was unacceptable to Hades, the god of the underworld,
who considered these souls his property.
 Hades
persuaded his brother Zeus, the king of gods, to hurl a lightning bolt
through Asklepios’ head. Zeus declared that medicine thereafter could
only be palliative, i.e., make patients more at ease while they either
died or got well on their own. Cures were forbidden.
Hades
persuaded his brother Zeus, the king of gods, to hurl a lightning bolt
through Asklepios’ head. Zeus declared that medicine thereafter could
only be palliative, i.e., make patients more at ease while they either
died or got well on their own. Cures were forbidden.
Given the
immortality of stem cells and their ability to cure and restore function
lost by disability, disease, or the entropy of aging, we may again incur
Zeus’ wrath as we develop their full healing potential. Although risking
being zapped to the Elysian Fields where Zeus’ victims were destined,
numerous innovators throughout the world are developing stem-cell
approaches to restore some function after spinal cord injury.
One of the more
promising approaches was first introduced in a
May 2003 article. That article
specifically described Portuguese neuropathologist Dr. Carlos Lima’s
procedures for implanting regenerative-endowed, stem-cell-rich,
olfactory tissue isolated from the patient’s nose into the spinal-cord
injury site.
The 5+ years of experience using these procedures
was recently reviewed at the 3rd International Symposium
for Olfactory Mucosa Autografts and Rehabilitation held May 9-10th
in Kefalonia, Greece – the birthplace of Asklepios,
Hippocrates, and Western medicine in general.
 (photo:
the authors in Kefalonia - L. Johnston, J. Peduzzi-Nelson, & C. Lima)
(photo:
the authors in Kefalonia - L. Johnston, J. Peduzzi-Nelson, & C. Lima)
In addition to the transplantation technique
itself, the meeting focused on how to best maximize restored function
through post-surgical, aggressive rehabilitation. Although the
transplantation procedures were developed in Portugal, many of Lima’s
patients have undergone post-operative rehabilitation in US facilities.
The insights gained at them are invaluable for not only assessing the
potential of Lima’s program, but also providing direction to other
function-restoring approaches that inevitably will be developed in the
future.
In this article, innovator Lima shares his insights
on important issues surrounding his program, and Dr. Jean Peduzzi-Nelson
will summarize for the first time supporting studies using the
procedures in rats.
Procedures
Because olfactory tissue is exposed to the air we
breathe, it contains cells with considerable turnover potential,
including renewable neurons, stem cells, and olfactory ensheathing cells
(OECs). Briefly, stem cells are progenitor cells that have the
potential to transform into CNS tissue, and OECs produce insulating
myelin sheaths around regenerating axons. Lima transplants whole
olfactory tissue because he believes that more than one cell type is
needed to maximize regeneration.
Lima’s Portuguese surgical team and international
colleagues have treated nearly 130 patients from throughout the world.
Many have reported functional recovery, ranging from the subtle to the
fairly dramatic. Recently, the World Technology Network named Lima as a
finalist for a prestigious innovation award in health and medicine.
A key procedure
is the collection of about one fourth of the patient’s olfactory tissue
through procedures that maximize the harvesting of that tissue but
avoids the collection of closely associated nasal respiratory
tissue. 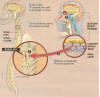 Because
the transplanted tissue is from the patient, immunological rejection is
minimized. The injury site is then surgically exposed, and
regeneration-blocking scar tissue is removed. The isolated olfactory
tissue is dissected into small pieces while immersed in the patient’s
cerebrospinal fluid. The pieces are then implanted into the cavity.
Because
the transplanted tissue is from the patient, immunological rejection is
minimized. The injury site is then surgically exposed, and
regeneration-blocking scar tissue is removed. The isolated olfactory
tissue is dissected into small pieces while immersed in the patient’s
cerebrospinal fluid. The pieces are then implanted into the cavity.
INSIGHTS ON USING OLFACTORY MUCOSA AUTOGRAFTS (OMA)
Carlos Lima, MD, Egas Moniz Hospital, Lisbon, Portugal
I’m focusing my
discussion on three key issues relevant to our OMA program: 1) the
post-injury disconnection syndrome; 2) the regeneration-blocking scar;
and 3) the use of whole olfactory tissue.
Disconnection
Syndrome
Because SCI represents
a disconnection syndrome, I believe functional recovery will require
extensive reorganization of the neural circuits surrounding the
injury site. This can be achieved through connection-building
cell-transplantation strategies (e.g., OMA) followed by functional
rewiring through post-operative, rehabilitation programs.
Studies suggest that
there is little regeneration of the long, movement-controlling
motor-neuron tracts that run down the spinal cord from the brain in the
mature nervous system. As expected, the injury alters the sensory input
that the brain receives from paralysis-affected body areas, which, in
turn, causes brain neural circuits to reorganize. Although this
reorganization promotes the death of injury-affected motor neurons
emanating from the brain, it stimulates the sprouting of spinal-cord
connective neurons (called propriospinal neurons) and the creation of
new, function-restoring circuits between them in incomplete injuries.
This means that the modified and newly created neural pathways will be
the ones responsible for mediating recovered function in patients with
incomplete injuries rather than the pathways that dominated before
injury.
To maximize restored
function after OMA-transplantation, we believe intense rehabilitation is
required - our ultimate goal being the patient relearning to walk.
Over-ground gait training with true weight bearing by the hips, knees
and feet is especially important and should stimulate or strengthen the
newly created neural pathways. Freedom of movement
unrestricted by braces will facilitate the development of new motor
patterns and functional connections, while hip and knee immobilization
may limit such development. Likewise, we should not restrict
rehabilitation to “normal” training patterns because “abnormal”
conditioning will probably be needed to re-establish motor skills that
now depend upon different nervous-system rewiring.
Scar
After injury, neural
support cells (e.g., astrocytes) and cells from neighboring connective
tissue (i.e., fibroblasts) generate a dense glial/connective scar at the
injury site, which is a barrier to regenerating neurons. Overall, from
the experience we have accrued using OMA procedures for chronic injury,
we have concluded that the scar is a “no-man’s land” with respect to
regeneration. Therefore, we believe it is important to remove it before
implanting enough regenerative-rich olfactory tissue to bridge both the
normal rostral and distal (i.e., top & bottom) stumps of the
spinal-cord. As our neurosurgical skills improve with experience,
combined with the capability to monitor through electrophysiological and
imaging methodology, the overall safety of scar removal will greatly
increase.
Cell Source
In
many severe injuries, the neuroplasticity or adaptability of residual
connections will be inherently limited without some kind of bridging and
rebuilding of nerve circuits. As such, it makes sense to transplant the
patient’s own olfactory tissue - endowed with regenerative
stem/precursor cells - into the patient’s injury site. We expect the
implanted olfactory tissue to mimic its natural properties of replacing
and repairing and will depend on Nature’s wisdom to do so when grafted
into the spinal cord. Using these implantation procedures combined with
rehabilitation, we believe we can bridge and re-establish
function-restoring neural connections after SCI.
Cumulatively, it is a long and challenging process, perhaps taking years
of effort to walk unassisted. But, nevertheless, it is an attainable
goal with hard work and a steadfast conviction of what is, indeed,
possible after injury. As Lao-Tsu stated: “A journey of a thousand miles
begins with a single step.”
INSIGHTS FROM ANIMAL STUDIES
Jean Peduzzi-Nelson, Ph.D., Wayne State University,
Detroit, Michigan
My laboratory evaluated Dr. Carlos Lima’s OMA
approach using a rat model of contusive spinal cord injury.
My early studies generated mysterious results.
Although transplants of either olfactory mucosa or bone-marrow-derived
stem cells improved locomotion in rats with chronic, severe SCI, the
combination of these two treatments resulted in much less improvement
than either treatment alone. This didn’t make sense because we thought
that such a combination would produce much better recovery. However,
more recent studies (supported by France’s ALARME foundation) solved the
mystery and provided further insight into the OMA mechanism.
OMA transplants produce significant functional
improvement as measured by “blinded” assessors who did not know what
treatment the rat received.

Interestingly, the olfactory-derived
stem/progenitor cells produced slightly more functional improvement.
For several years, experts have assumed Lima’s clinical results were due
to the OECs within the olfactory tissue. Indeed, this OEC emphasis has
been embraced by Australian and UK researchers. However, our results
strongly suggest that it is the stem-cells and not the OECs that
are primarily responsible for functional improvement. Long suspected by
Lima, it justifies his use of whole olfactory tissue rather than merely
the OECs isolated from such tissue.
Another fascinating result was that although the
stem cells produced greater functional improvement, it did not reach
statistical significance. The reason behind this and probably the
reason that the previous combination treatment failed was that stem
cells that are not from the same animal or an animal that is closely
tissue matched (similar to blood matching) are not as effective.
In the latest study, the various treatments were
done in both inbred (closely matched – similar to a transplant from a
sister or brother that have the same blood and tissue type) and outbred
strains (similar to a transplant from a cousin that may not be of the
same blood and tissue type). Each inbred strain received cells or
tissue from the same inbred strain, and each outbred strain received
cells or tissue from the same outbred strain.
Results indicated that functional improvement was
obtained when either olfactory or bone-marrow-derived stem cells are
used in the inbred strain but not when used in an outbred strain. It
means that stem cells from the same individual or a closely related
matched individual might cause functional improvement, while cells or
tissue from a distantly related individual would not. The reason could
be that the stem cells that are foreign do not grow and mature as well,
and therefore do not cause functional improvement. There may
also be some rejection of the foreign cells. Although stem cells are
generally not rejected, when the stem cells from a distantly related
animal mature, they may acquire characteristics that the recipient
considers foreign.
Now the mystery is solved concerning the
combination treatment. Most likely, by combining two treatments in an
outbred strain, it produced a greater rejection of the cells as
foreign. In conclusion, our findings support 1) the idea that
olfactory-derived stem cells are responsible for the improvement after
using function-restoring OMA procedures, and 2) the importance of using
one’s own stem cells for functional recovery.
Adapted from article appearing in August 2008 Paraplegia News (For subscriptions,
call 602-224-0500) or go to
www.pn-magazine.com.
TOP