In a season-opening game, Buffalo Bills tight end
Kevin Everett sustained a cervical C3-4 injury from tackling an
opponent. While still in the ambulance, an ice-cold saline solution was
injected into Everett putting him into a neuroprotective, hypothermic
state. He soon regained significant function, which may (or may not)
have resulted from the cooling.
Cooling the acutely injured cord is not a recent
development, and, as reviewed by Miami-Project investigators Drs.
Alberto Martinez-Arizala and Barth Green (1992) and Drs. James Guest and
Dalton Dietrich (2005), various permutations have been tried over the
years. Observations dating back to the 1950s suggest that lowering
central-nervous-system (CNS) temperature can mitigate the harmful
effects of restricted blood flow and oxygen deficiency during, for
example, CNS-blood-flow-disrupting operations. Based on the
observations, hypothermic cooling procedures were developed to save
neurological function after acute spinal cord injury (SCI).
Supposedly, the procedures protect the injured cord
by reducing its metabolic and energetic requirements. Like putting the
injured neurons on life support, they donít need as much viable cellular
processes to keep functioning in the cooled state and, therefore, may
survive longer. Similar to an ice-pack on a sprain, cooling reduces
neuron-damaging, injury-site swelling and bleeding.
Although SCI studies suggest intriguing potential,
care must be taken in over-generalizing results in humans because the
studies 1) involved limited cases, 2) lacked controls, 3) reported only
generalized improvement during a period in which some gain is not
unusual, 4) varied in the time from injury to when cooling was started,
and 5) were potentially confounded by the concomitant use of other
treatments.
Although the neuroprotective potential of cooling
is being revisited, most clinical experience was acquired in the
1960-70s. By the 1980s, enthusiasm cooled off because of ambiguous
results, technical complexity, and decreased use of emergency
laminectomy needed to expose the injured cord to cooling. [laminectomy
removes function-compromising, cord-compressing tissue or bone
fragments].
Essentially, procedures can be categorized as
either systemic (i.e., whole body) hypothermia or localized cooling:
LOCALIZED COOLING
1) Dr. Gaston Acosta-Rua (Iowa City, IA) treated
two men (17 & 21) with thoracic injuries from motor-vehicle accidents
with spinal-cord cooling (1970). After a decompressive laminectomy, the
cordís outer membrane was opened, and the cord cooled for three hours
with recirculated, ice-cold saline. The time from injury to cooling was
two days in the first case and several hours in the second. Both
patients improved.
2) Dr. Y. K. Demian et al (Cleveland, OH) treated
three patients (age 15, 17, & 18) with cervical injuries (1971). After
laminectomy, the cord was cooled for 1.5 - 3 hours with ice-cold saline.
In two cases, the time from injury to cooling was about five hours; in
one, it was over 12 hours. Recovery was noted in all.
3) Dr. Robert Selker (Chicago, IL) used hypothermic
cooling to treat four acutely injured patients (2 cervical & 2 thoracic;
3 from gunshot) within three hours of injury (1971). Although two died
several months later, the other two regained some function.
4) Dr. Dexter Koons and colleagues (Tucson, AZ)
treated five patients with cervical (2) and thoracic (3) inj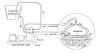 uries
with hypothermic procedures (1972). After a decompressive laminectomy
3-7 hours after injury, the cordís outer membrane was opened, and the
cord was cooled with a saline slush for 30 minutes. Most patients did
not regain function.
uries
with hypothermic procedures (1972). After a decompressive laminectomy
3-7 hours after injury, the cordís outer membrane was opened, and the
cord was cooled with a saline slush for 30 minutes. Most patients did
not regain function.
5) Drs. William Meacham and Warren McPherson
(Nashville, TN) treated 14 patients with spinal-cord cooling within
eight hours of injury (1973). Age ranged from 16 to 56; all but three
were male; and 12 and 2 had cervical and thoracic injuries,
respectively. After a wide decompressive laminectomy, the cord was
cooled by cold saline for three hours. Four patients died. Of the 10
survivors, seven had some improvement, including improved sensation,
motor control, and bladder functioning.
6) Dr. Juan Negrin (New York, NY) treated three
patients with delayed cooling (1975). With the first patient, who
sustained a thoracic injury five hours before laminectomy, the cord was
cooled without opening its membrane for three, 45-minute periods two,
three, and four days after surgery. No improvement was reported. With
the second patient, who had a laminectomy a day after injury, t he
cord was cooled for one hour after opening the covering membranes.
Several weeks later when the cord needed to be opened once more, cooling
was carried out again for another hour. The patient regained
considerable function. Due to delayed complications, the third patientís
decompressive laminectomy was undertaken a year after acquiring a
cervical injury. After opening the covering membranes, cooling was
carried out for 45 minutes. Improvement was noted.
he
cord was cooled for one hour after opening the covering membranes.
Several weeks later when the cord needed to be opened once more, cooling
was carried out again for another hour. The patient regained
considerable function. Due to delayed complications, the third patientís
decompressive laminectomy was undertaken a year after acquiring a
cervical injury. After opening the covering membranes, cooling was
carried out for 45 minutes. Improvement was noted.
7) Dr. Charles Tator (Toronto, Ontario) irrigated
the acutely injured spinal cord of 11 patients (7 cervical & 4 thoracic;
age 16-56) with either cooled or body-temperature solutions (1979). He
suggested that non-cooling irrigation still provides benefits because
the solution provides oxygen to the injured tissue, creates a
biochemically supportive environment, and flushes out noxious
substances. The time from injury to surgery varied from 3-8 hours. The
irrigations were carried out with covering membranes widely opened.
Three patients recovered some sensation, one of whom also regained some
motor function (toe wiggling).
8) Dr. Robert Hansebout and colleagues (Hamilton,
Ontario) treated seven males and three females (6 thoracic and 4
cervical injuries) within 8.5 hours of injury (1984). After
decompression, a cooling saddle was placed lightly against the cordís
outer membrane for four hours. Followed for at least six months, three
patients accrued some motor or sensory recovery (one died).
SYSTEMIC COOLING
Due to a rise in body temperature after injury,
Everett was systemically cooled, a procedure that has been more
extensively used in traumatic brain injury (TBI). Even with TBI,
however, the benefits of such cooling have been ambiguous. For example,
a study completed in 1998 involving 392 patients with TBI did not
demonstrate significant benefits, except in younger, quickly treated
patients.
For SCI, medications used to prevent shivering
interfere with monitoring neurological function and promote health
complications. Addressing this issue, Dr. Jogi Inamasu and colleagues
(2003; Tokyo, Japan) stated ďÖ patients with cervical SCI, who are most
vulnerable to respiratory infection, hypotension, and bradycardia [slow
heart rate] may be further compromised by induction of systemic
hypothermia,Ē further noting that the prolonged use of sedatives and
muscle relaxants essential during systemic hypothermia may worsen the
respiratory function of these ďfragile patients.Ē
Nevertheless, because animal studies suggest that
post-injury elevated body temperatures are detrimental, Miami-Project
investigators have started using state-of-the-art technology to treat
acutely injured patients with mild hypothermia, producing a
several degree drop in body temperature. Basically, a catheter is placed
in the patientís blood vessel, and a thermo-regulating device closely
monitors and adjusts blood temperature as it passes by the catheter.
The study will follow long-term benefits by assessing improvements in
motor and sensory function and acquisition of daily-living skills.
CONCLUSION
The still-to-be-defined neuroprotective benefits
associated with cooling the acutely injured spinal cord must be
carefully weighed relative to potential risks. Because the results of
various animal and human studies have been ambiguous and often
contradictory, more definitive studies are needed.
Adapted from article appearing in December 2007 Paraplegia News (For subscriptions,
call 602-224-0500) or go to
www.pn-magazine.com.
TOP